The Secret Life of Sildenafil (Viagra) revealed by AI

What if Sildenafil (Viagra) could do even more than what we already know?
Today, over 95% of diseases do not have an approved treatment. Because of the cost (2 billion USD on average) and time (10 years) involved in developing a new drug, researchers have looked into repurposing some of the 3000 existing drugs on the market to treat diseases for which they weren’t originally approved. Sadly, identifying the right drug for the right disease is extremely difficult. This difficulty is partially due to how complex it is to identify which proteins these drugs interact with to induce their effect (their “mechanism of action”), and to link this mechanism to a disease. The discovery of a single mechanism of action usually requires years of fundamental research, and serendipitous discoveries are far more common than those originating from concerted efforts. To further complicate the problem, drugs often do not have a single mechanism of action, but many different ones.
As part of an effort to accelerate the repurposing of existing drugs for new indications, Kantify has used Artificial Intelligence to evaluate possible mechanisms of action of over 78,000 repurposable or natural drugs with its novel AI platform, Sapian. This effort resulted in more than 1.4 billion screened interactions - one of the largest AI drug screening to date. Thanks to this first of its kind AI drug screening, the company is now able to identify novel ways of using known drugs.
Through this first issue of the “Behind the Pill” Series, Kantify shares the predictions of its AI platform Sapian for a well known drug, Sildenafil, also known as Viagra. While Sildenafil is known for its role in treating erectile dysfunction and pulmonary arterial hypertension, there have been reports of various biological effects of this drug, including a potential role in preventing Alzheimer’s disease, mitochondrial complex V deficiencies and cancer, to name a few. Nevertheless, despite significant efforts and research, the mechanisms through which Sildenafil induces these diverse effects have largely remained unknown. In this work, Sapian predicts that the drug likely interacts with over 245 proteins to cause its wide-ranging biological effects. Amongst these 245 proteins, the AI identifies groups linked to key biological functions: mitochondrial function, gaba and other neuroreceptors, and even autophagy, further opening up the doors to use this drug in diseases known to have dysfunctions in these processes. Many of these proteins have never been associated to Sildenafil before.
Through the example of Sildenafil, Kantify demonstrates that AI can help uncover many mechanisms of drugs, leading to new uses of existing drugs, and the development of targeted therapies.
A Peek Behind the Pill
At Kantify, we've embarked on an extraordinary journey to demystify the actions of well-known drugs, starting with a name that's both famous and infamous: Sildenafil, better known by its trade name, Viagra. Sildenafil is a widely prescribed small molecule drug - in 2021, it was the 157th most commonly prescribed medication in the United States , with more than 3 million prescriptions.

Viagra™ in its iconic “blue pill” form. Image courtesy of Wikimedia.
Sildenafil's primary uses in treating erectile dysfunction (ED) and pulmonary arterial hypertension (PAH) are well-documented. However, recent studies have shown promising effects of this drug on preventing Alzheimer’s disease¹, indicating that this drug has wide-ranging health effects through mechanisms that are currently poorly understood. Furthermore, while the reported mechanism of action involves inhibiting a protein called phosphodiesterase type 5 (PDE5), several studies have shown that Sildenafil affects other protein targets². As such, one may assume that the larger therapeutic potential of Sildenafil may be related to pharmacological actions yet to be discovered.
Given its popularity and wide-ranging health effects, advancing the understanding of Sildenafil’s Mechanism of Action (MoA) has important consequences. Firstly, discovering these MoAs could result in new uses of Sildenafil to treat more diseases. Secondly, the discovery of new MoAs could drive the development of first-in-class drugs that selectively leverage some of the mechanisms that Sildenafil uses, with fewer risks and side effects.
The Complexity of Deciphering Drug Actions
Understanding a drug's mechanism of action (MoA) is a complex challenge, primarily because drugs often interact with multiple targets within the body, not just the ones they are initially designed to influence. This polypharmacology, also known as “off target effects” is often not fully understood for most small molecule drugs. It means that a single drug like Sildenafil can inadvertently affect various biological pathways and cellular processes. This may contribute to both its beneficial therapeutic effects and unwanted side effects. Moreover, these interactions can be subtle and involve changes at the molecular level that are not easily detectable with standard assays considering the size of the human proteome. At Kantify, our mission is to decode these complex biological interactions using cutting-edge AI algorithms, allowing us to identify and understand the subtle effects drugs can have within the body.
Traditional techniques for studying drug mechanisms are often limited in their scope and may overlook the broader biological context in which a drug operates. These methods typically focus on predefined drug targets and are not equipped to detect unexpected off-target effects or interactions between multiple pathways, which are crucial for understanding a drug’s full spectrum of action. Additionally, these conventional approaches can be time-consuming and require extensive resources, which may not be feasible for all research institutions or companies. As a result, there is a significant gap in our ability to fully comprehend the complex dynamics of drug actions, necessitating the development of more holistic and integrated approaches to drug discovery and analysis.
At Kantify, we are leveraging our groundbreaking machine learning (AI) model, Sapian , to revolutionize how drug interactions are predicted and understood. After extensive training, Sapian has learned to accurately predict interactions between any small molecules and any protein target. This remarkable capability of Sapian to perform across an extensive range of molecules and targets makes it an invaluable tool in the field of drug discovery. Utilizing Sapian, we conducted an extremely large throughput virtual screening, evaluating 78,000 repurposable or natural drugs—including Sildenafil—against over 18,000 different human proteins. In other words, we use our machine learning model to predict the protein targets of 78 000 drugs. This comprehensive screening produced over 1.5 billion individual results, marking it as one of the largest virtual screenings ever undertaken.
Unveiling New Dimensions in Sildenafil's Potential
In order to visually capture the complex potential of Sildenafil, we’ve generated an atlas of the human proteome, as learned by Sapian. This atlas displays the human proteome, mapped in a 2-dimensional space. Unlike a traditional geographical atlas, where the Y-axis represents the North to South prime meridian, and the X-axis represents the East to West equator, our atlas’ axes do not have a similar meaning. Instead, rather than absolute location, what matters in our map is proximity. More specifically, when proteins are close to one another, Sapian manages to see them as being similar - in terms of structure, drugability, biological function, or other important features. On the atlas below, where each dot represents a human protein, we display PDE5, the putative target of Sildenafil, together with its closest neighbors. We observe that these neighbors are indeed other proteins of the same family, such as PDE6A&B&C, PDE2A and PDE11A - a remarkable feat, as the regrouping of proteins in similar families is often a painstaking work resulting from years of research by experts, which Sapian has seemingly learned on its own.
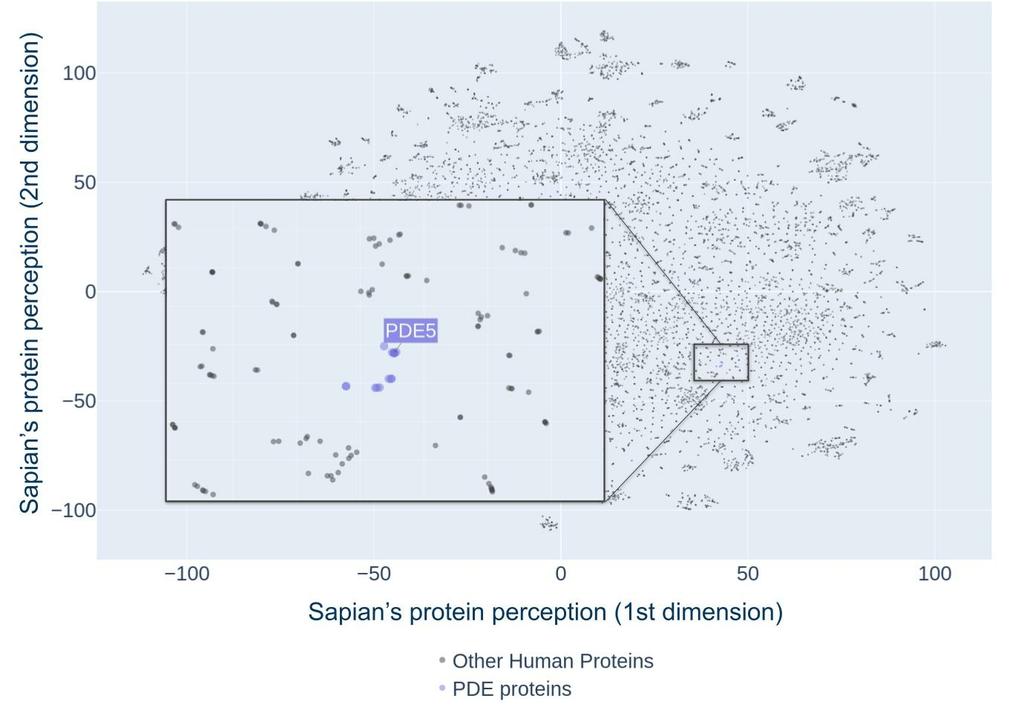
The learned representation of Sapian of the human proteome (Kantify’s Target Atlas). Each dot represents one single protein in the human proteome. A cutout focuses on PDE proteins - the putative target family of Sildenafil.
Next, we can zoom out over the whole proteome, and overlay a heatmap to show which proteins Sildenafil is most likely to interact with. In this map, warmer colors indicate a higher predicted likelihood that Sildenafil interacts with proteins in this space of the Atlas. This visual format not only highlights key areas with dense interactions but allows us to identify specific clusters of proteins that have not been extensively studied in relation to Sildenafil. These highlighted areas suggest new possibilities for research, encouraging us to delve into specific protein interactions that might reveal new uses for Sildenafil beyond its known applications. Additionally, we've cross-referenced our predictions with existing scientific literature to verify if any of the predicted target interactions could be supported by already observed effects. This validation step is crucial as it anchors our predictions in real-world biological functions and helps refine our hypotheses.
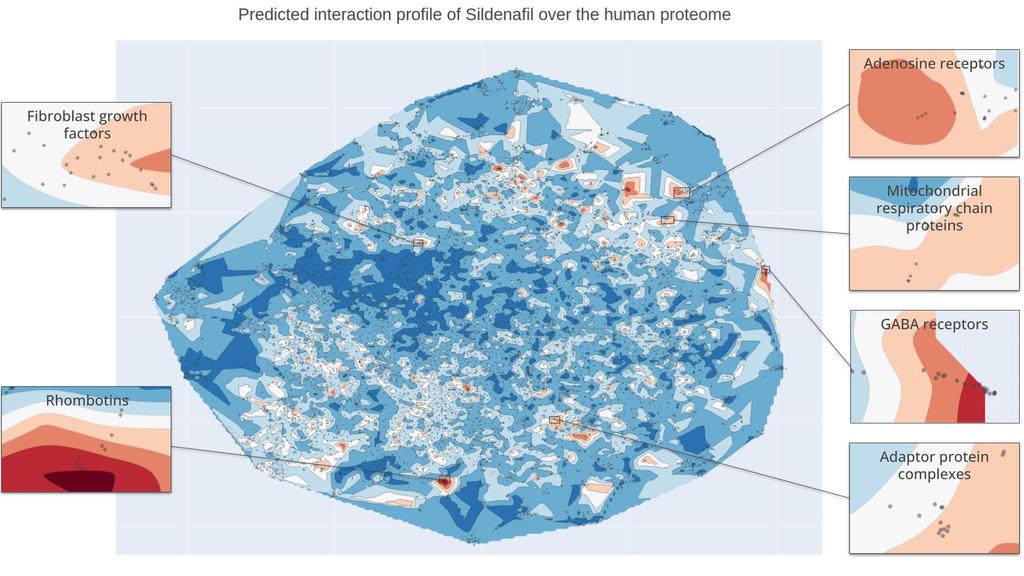
Kantify’s Target Atlas for Sildenafil. Warm zones indicate a higher likelihood that Sildenafil interacts with these groups of families. Cutouts focus on 6 families that we detail below.
Expanding the Horizon: Sildenafil's Broader Interactions
Despite most of the proteome showing negligible interaction with Sildenafil, our analysis with Sapian pinpoints potential interactions with approximately 245 protein targets, including but extending beyond its well-documented phosphodiesterase (PDE) targets. This highlights a vast, intricate network of influences, suggesting that Sildenafil may exert its therapeutic and side effects through a complex polypharmacological profile. To go one step further, we used string-db for network analysis, therefore highlighting a large intricate network of protein interactions (physical, co-occurrence, co-expression, gene fusions) within our predicted targets of Sildenafil, as visible in the below graph. This network was further clustered to identify groups of highly interrelated proteins, and for each cluster - identified by a node color on the graph- pathway augmentation analysis was performed to better understand the underlying biological functions Sildenafil could be modulating. In the following sections, we highlight our main findings.
In a nutshell, Sapian proposes new hypotheses for further repurposing of Sildenafil, which will have to be validated in a wet lab.
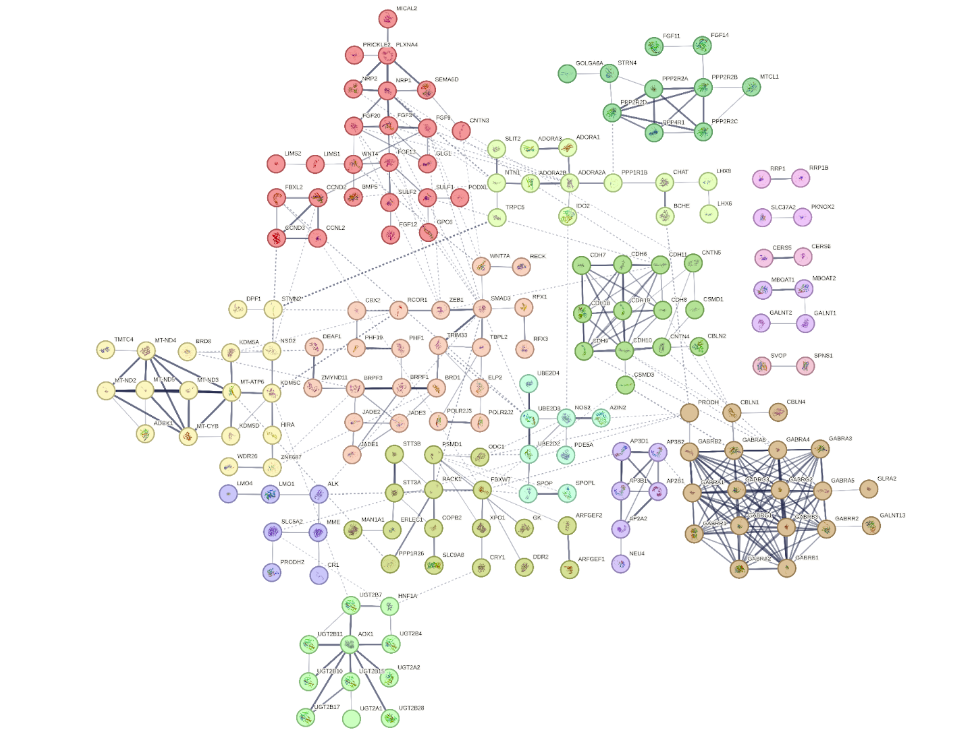
StringDB Network analysis of the top 245 targets identified by Sapian for Sildenafil. Lines indicate protein interactions. Colors indicate clusters of interactions.
Our cluster analysis of sildenafil’s predicted targets using string-db highlights GABA receptor proteins as crucial mediators, suggesting their involvement in various biological and neurological processes. This cluster is associated with pathways such as cellular response to histamine, GABAergic signaling, and synaptic transmission, which are linked to conditions like schizoaffective disorder, epilepsy, and substance-related disorders. The role of histamine in penile erection³ suggests a psychoactive mechanism of sildenafil that could improve erectile functions and arousal. Furthermore, the identified GABAergic pathways overlap with morphine⁴, nicotine and cannabinoid pathways, aligning with studies showing that these substances significantly modify exposure to sildenafil’s⁵. Additionally, the relevance of GABA signaling in Alzheimer's disease has been reinforced by recent reviews, advocating for its consideration in developing therapeutic strategies⁶. This comprehensive analysis not only supports sildenafil's effectiveness in treating psychogenic erectile dysfunction but also highlights its potential repositioning for treating neurological disorders.
Our predictions also suggest that sildenafil influences a group of mitochondrial proteins within the respiratory chain, known as the respirasome. These proteins are extensively documented in connection with neurodegenerative disorders such as Huntington's, Alzheimer's, and Parkinson's diseases. There is compelling evidence in the literature indicating that sildenafil may help rescue mitochondrial functions, supporting its potential repositioning for these diseases. Moreover, these mitochondrial proteins have been specifically associated with Leigh disease and other mitochondrial diseases, for which sildenafil has been identified as a promising treatment⁷. This association is particularly significant given the severe mitochondrial dysfunction observed in Leigh disease, suggesting that sildenafil’s mechanism may extend to modifying disease progression at a mitochondrial level. Furthermore, these mitochondrial targets are associated with low blood levels of citrulline. Citrulline is particularly interesting in the context of erectile dysfunction⁸, as a precursor for nitric oxide synthesis, which is required to achieve erections. This biochemical pathway highlights a potential comprehensive mechanism of action for sildenafil, possibly explaining how sildenafil's effects on the mitochondrial level could contribute to its overall polypharmacological profile even in its use for erectile dysfunction.
We also predict that Sildenafil modulates Adenosine receptors as well as Fibroblast Growth Factors (FGFs). Adenosine receptors⁹ and FGFs¹⁰ were already identified as important therapeutic targets in the context of Pulmonary Arterial Hypertension (PAH), Sildenafil’s second FDA approved indication. This association again suggests the existence of a polypharmacological mode of action which aligns with its clinical use in improving pulmonary vascular resistance and patient status in pulmonary fibrosis¹¹. Sapian also predicted an interaction with Sulfatases, a family of proteins known to play a role in esophagus smooth muscle contraction. Surprisingly, it was reported that Sildenafil relieves symptoms and normalizes motility in patients with esophageal spasm¹², an observation nicely fitting our prediction. Lastly, the predicted connection to autophagy proteins, such as Adaptor protein complexes, correlates with literature¹³ and positions Sildenafil as a promising agent in neuroprotection and managing protein aggregation disorders, expanding the conversation on its therapeutic scope.
A Call to Reimagine Drug MoAs
Our findings advocate for a more expansive view of drug mechanisms, moving beyond the traditional single-target paradigms to embrace the complexity inherent in polypharmacology. Sildenafil, with its myriad interactions uncovered, exemplifies the need for this paradigm shift in drug discovery. Not only can we better explain the current usages of this molecule, but we also hope to launch new efforts to expand its use in treating more diseases.
At Kantify, we are not just predicting interactions; we are uncovering the narratives hidden within every molecule. Our journey with Viagra is merely the beginning. We invite clinicians, biologists, and the pharmaceutical community to join us in this exploration, to challenge existing knowledge and discover the full potential of drugs we thought we understood.
Behind the Pill is more than just a series of publications; it is a gateway to understanding the hidden complexities of the medications we use.
Follow us next month as we delve into another drug's secret life.
Together, we might uncover surprising new insights.
Learn more
For inquiries or suggestions on which drug to explore next, reach out to us at Kantify. Let’s embark on this quest together, to decipher the secrets embedded in the very fabric of pharmacology.
Curious about the off-targets of a small molecule that is in clinical trials or on the market? Get in touch, or follow us online to receive our next target Behind the Pill article.
Addendum
Below is the list of novel predicted targets for Sildenafil, ordered by descending strength of signal.
LMO4, GLG1, KDM5A, LMO3, GABRR2, LMO4, PRPSAP2, CSMD3, KDM5A, GABRG2, GALNT9, GABRB3, SLC37A1, BRPF3, GABRA5, BRPF1, LMO1, GLG1, GABRG3, SPOP, BRPF3, GABRR1, BRD1, CSMD1, PHF1, GABRA2, PHF1, JADE1, GABRB2, FGF14, LIMS1, DDR2, FGF12, MBOAT2, CSMD3, GLRA2, GLRA1, CBLN1, PPP2R2A, GLRA3, GABRA3, SULF1, PHF19, CERS5, UGT2B4, ADORA2B, SLIT2, GABRG1, BRD8, RRP1B, RFX3, TBPL2, FGF13, CDH9, JADE2, SPOPL, GABRA6, CDH10, TMTC4, SLIT2, PPP2R2B, CSMD2, LMO1, BMP5, CDH10, UGT2A2, GPC6, SLK, ITGAV, NSD2, UGT2B15, LMO3, CSMD1, UGT2B17, CBLN2, PPP2R2C, JADE2, AP3D1, ARFGEF1, UGT2B7, BRD1, RRP1, PRPSAP2, CDH9, CDH11, PIGN, GALNT2, PIGN, ADORA2A, BRPF1, NTN1, ANKIB1, NSD2, ZBTB8A, GABRB1, PPP2R2D, JADE1, ENPP2, SMAD3, FBXL2, NRP2, RFX1, CR1, MAN1A1, GALNTL6, NELL1, GALNT13, LHX6, PHF19, ELP2, PPP2R2A, PCSK6, RCOR2, MT-ND4, GABRA1, SULF2, CPXM2, DPF1, FGF13, PKNOX2, MME, NTN1, MT-CYB, AOX1, SLC8B1, STMN2, CBLN1, PRICKLE2, PPP2R2D, ADCK1, SUCO, ZFTA, ATRNL1, SLK, PRODH2, POLR2J3, NEU4, UBE2D3, PPP1R1B, CDH19, RFX3, FGF9, ADORA3, MAN1A1, TATDN2, ICMT, CDH7, CPT1A, POLR2J2, ADORA1, CDH18, PRICKLE2, CCND2, CCNL2, CCND3, MBOAT1, CHAT, GALNT9, ZEB1, ABI3BP, ADIPOR2, CERS6, ZMYND11, XPO1, LIMS1, GK, SPOP, COPB2, CBLN4, GABRA4, FBXW7, RCOR1, ONECUT3, UBE2D2, SF3B3, UGT2B10, CCDC30, LSP1, ITPKB, ATRN, RACK1, PODXL, BCHE, GOLGA6B, SVOP, STT3B, LIMS2, AP3B1, WDR26, ALG9, ACSM5, ZNF687, ZBTB12, SLC5A2, UGT2A1, GK3, UGT2B11, GALNT5, MT-ND3, STT3A, SLC9A8, PCSK6, UGT2B28, NPNT, NRP1, SEH1L, ADCK1, NOS2, MT-ND2, JADE3, WNT7A, SLC9A4, NETO2, CSMD2, TLL1, AP3B2, AP2A2, SLC37A2, C10orf90, ABI3BP, ARFGEF2, CCDC9, MICAL2, AP2B1, TRPC5, TMPRSS15, FGF14, MT-ND5, CRY1, MT-ATP6, FGF3, ALK, LHX8, STT3B, PDE3B, CNTN3, NRP2, GALNT1, STRN4, AZIN2, CDH6, CDH8, RECK, CHRNB3, DEAF1, FBXL2, GOLGA6A, MTCL1, PPP4R1, CNTN5, UBE2D4, UPRT, SEMA6D, HNF1A, FGF11, MLPH, AP3B1, FGF20, CR1L, ARHGAP30, FSCN2, SLC37A1, PLXNA4, WDR26, PODXL, PAG1, ZNF827, NELL1, DYNLRB2, ERLEC1, SULF1, COPB2, PDK1, IDO2, FGF11, MT-ND4, HIRA, CCND2, MT-ND2, WNT4, SPNS1, KDM5C, KDM5D, YY1AP1, ALK, WIZ, CNTN4, PSMD1, RAD52, CBX2, KIF2B, ODC1, KLHL4, KDM4C, PPP1R26, FSCN2, CDH8, GALNT13, PRODH, TRIM33
Disclaimer
The information provided in this article is for informational purposes only and is not intended as medical advice. This content should not be used to diagnose, treat, cure, or prevent any medical condition. Always consult a qualified healthcare provider for advice regarding any medical concerns or before starting or stopping any medication. The insights and findings discussed are based on research and are not a substitute for professional medical guidance.
Authors
Nicolas Maignan, David Papazian, Maxime Georges, Jack Dawe, Caio Hudson de Souza, Rubal Ravinder, Ségolène Martin, Nik Subramanian
About Viagra
Viagra® (sildenafil citrate) is a drug originally developed by Pfizer Inc.
Sources
[1] Gohel D, Zhang P, Gupta AK, Li Y, Chiang CW, Li L, Hou Y, Pieper AA, Cummings J, Cheng F. Sildenafil as a Candidate Drug for Alzheimer\'s Disease: Real-World Patient Data Observation and Mechanistic Observations from Patient-Induced Pluripotent Stem Cell-Derived Neurons. J Alzheimers Dis. 2024;98(2):643-657. doi: 10.3233/JAD-231391. PMID: 38427489; PMCID: PMC10977448.
[2] Balaji S, Patnaik YR, Surin WR. Off-target effect of high-dose sildenafil on adenosine 5\'- diphosphate and collagen-induced platelet activation through mitogen-activated protein kinase pathway in treated BALB/C mice and in vitro experiments: A preliminary study. Indian J Pharmacol. 2024 Mar 1;56(2):136-140. doi: 10.4103/ijp.ijp_312_23. Epub 2024 Apr 30. PMID: 38808925; PMCID: PMC11161001.
[3] Cará AM, Lopes-Martins RA, Antunes E, Nahoum CR, De Nucci G. The role of histamine in human penile erection. Br J Urol. 1995 Feb;75(2):220-4. doi: 10.1111/j.1464-410x.1995.tb07315.x. PMID: 7850330.\n\n[4] Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003 Mar;67(3):150-6. doi: 10.1159/000067802. PMID: 12571411.
[5] Murtadha M, Raslan MA, Fahmy SF, Sabri NA. Changes in the Pharmacokinetics and Pharmacodynamics of Sildenafil in Cigarette and Cannabis Smokers. Pharmaceutics. 2021 Jun 13;13(6):876. doi: 10.3390/pharmaceutics13060876. PMID: 34199328; PMCID: PMC8231986.
[6] Carello-Collar G, Bellaver B, Ferreira PCL, Ferrari-Souza JP, Ramos VG, Therriault J, Tissot C, De Bastiani MA, Soares C, Pascoal TA, Rosa-Neto P, Souza DO, Zimmer ER. The GABAergic system in Alzheimer's disease: a systematic review with meta-analysis. Mol Psychiatry. 2023 Dec;28(12):5025-5036. doi: 10.1038/s41380-023-02140-w. Epub 2023 Jul 7. PMID: 37419974.
[7] Bottani E, Lamperti C, Prigione A, Tiranti V, Persico N, Brunetti D. Therapeutic Approaches to Treat Mitochondrial Diseases: "One-Size-Fits-All" and "Precision Medicine" Strategies. Pharmaceutics. 2020 Nov 11;12(11):1083. doi: 10.3390/pharmaceutics12111083. PMID: 33187380; PMCID: PMC7696526.
[8] Cormio L, De Siati M, Lorusso F, Selvaggio O, Mirabella L, Sanguedolce F, Carrieri G. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology. 2011 Jan;77(1):119-22. doi: 10.1016/j.urology.2010.08.028. PMID: 21195829.
[9] Alencar AKN, Montes GC, Barreiro EJ, Sudo RT, Zapata-Sudo G. Adenosine Receptors As Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front Pharmacol. 2017 Dec 4;8:858. doi: 10.3389/fphar.2017.00858. PMID: 29255415; PMCID: PMC5722832.
[10] Woo KV, Shen IY, Weinheimer CJ, Kovacs A, Nigro J, Lin CY, Chakinala M, Byers DE, Ornitz DM. Endothelial FGF signaling is protective in hypoxia-induced pulmonary hypertension. J Clin Invest. 2021 Sep 1;131(17):e141467. doi: 10.1172/JCI141467. PMID: 34623323; PMCID: PMC8409583.
[11] Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007 Mar;131(3):897-899. doi: 10.1378/chest.06-2101. PMID: 17356110; PMCID: PMC2098039.
[12] Fox M, Sweis R, Wong T, Anggiansah A. Sildenafil relieves symptoms and normalizes motility in patients with oesophageal spasm: a report of two cases. Neurogastroenterol Motil. 2007 Oct;19(10):798-803. doi: 10.1111/j.1365-2982.2007.00957.x. PMID: 17883431.
[13] Duarte-Silva E, Meiry da Rocha Araújo S, Oliveira WH, Lós DB, Bonfanti AP, Peron G, de Lima Thomaz L, Verinaud L, Peixoto CA. Sildenafil Alleviates Murine Experimental Autoimmune Encephalomyelitis by Triggering Autophagy in the Spinal Cord. Front Immunol. 2021 May 13;12:671511. doi: 10.3389/fimmu.2021.671511. PMID: 34054847; PMCID: PMC8156813.